The sponsor shall submit an unique and two copies of all submissions into the IND file, including the original submission and all amendments and studies.
(ii) Oblique prices consist of charges incurred generally to produce the drug for professional sale (e.g., charges for facilities and products accustomed to manufacture the supply of investigational drug, but which can be primarily meant to produce big quantities of drug for eventual professional sale) and analysis and development, administrative, labor, or other prices that may be incurred although the scientific demo or cure use for which charging is approved didn't take place.
Just as critical for us was qualifying that site visitors, considering the fact that we do written content composing, not in-depth duplicate producing. So, we provided a match / it’s not a fit portion that, in a very playful but insightful way, allowed visitors to self segment so we didn’t overwhelm our revenue workforce with sales opportunities that were trying to find what we couldn’t produce.”
This presentation is about The fundamental obligations and features of CDSCO explaining the regulatory physique's Structure, comprising of functions of condition licensing authority and port offices covering the guidelines for new drug approval approach, scientific trails and health-related devices. this presentation also give a simple note on SUGAM
FDA acknowledges that modifications to the strategy of preparing of The brand new drug substance and dosage type and variations inside the dosage variety by itself are possible since the investigation progresses. Hence, the emphasis within an initial Phase one submission must frequently be placed on the identification and control from the Uncooked elements and The brand new drug substance. Last specs with the drug compound and drug product are not anticipated till the end in the investigational method.
The FDA has thirty days to assessment an IND for protection ahead of trials may start. Clinical retains can be placed on applications that pose unreasonable threats or are missing essential information. Notifications are provided to sponsors concerning evaluate results and any deficiencies that should be resolved.
It's going to take a few 12 months to evaluation an NDA and various forms and fees are involved in the approval and import license application procedures.
ICH has developed several tips on quality, protection, efficacy and multidisciplinary topics that happen to be applied by regulatory organizations in ICH areas and used globally to streamline drug improvement and approval procedures.
In the Acceptance Assessment, the Guide Reviewer decides if the 510(k) submission fulfills the minimum amount threshold of acceptability and may be recognized for substantive overview.
How it performs: “We built some modifications on this page a number of months in the past after obtaining insights from our A/B exams, heatmaps, and analytics. Before you make Abbreviated New Drug Application (ANDA) improvements the landing webpage had about 5%-6% conversion price, but as soon as we designed the improvements relevant to style, content material, and CTAs, Our conversions boosted repeatedly.
A sponsor shall post a protocol amendment for the new protocol or maybe a modify in protocol in advance of its implementation. Protocol amendments so as to add a new investigator or to deliver added information about investigators could possibly be grouped and submitted at 30-working day intervals.
Also, We've got delivered all the info that a consumer desires to move forward even further. Proper CTAs at the ideal positions and our unbeatable determination will help us to convert more and more people.”
Gurjar Pratihara Dynasty has a great great importance in historical Indian record. This dynasty made An effective effort and hard work to re-set up the political unity of northern India which was shattered following the death of Harsha. Its mighty kings retained the vast majority of northern India beneath their control for a long period.
A sponsor shall report within an details Modification important information on the IND that isn't in the scope of a protocol Modification, IND basic safety experiences, or yearly report. Examples of knowledge demanding an information and facts Modification include things like:
 Daniel Stern Then & Now!
Daniel Stern Then & Now!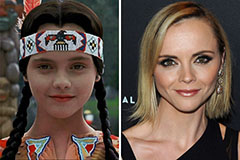 Christina Ricci Then & Now!
Christina Ricci Then & Now! Barry Watson Then & Now!
Barry Watson Then & Now! Sam Woods Then & Now!
Sam Woods Then & Now! Katey Sagal Then & Now!
Katey Sagal Then & Now!